About Event
Please be aware that this event has been cancelled.
The meeting serves as the definitive forum for predicting, monitoring, and mitigating adverse immune responses to AAV gene therapies, designed to equip you with the tools and insights needed to accelerate your programs safely and effectively.
Sessions will dive deep into the most pressing topics, including navigating complement activation and biodistribution to mitigate immune risks while ensuring precise, targeted delivery. Explore strategic insights into repeat dosing and immune management, equipping you to overcome barriers and extend the durability of therapeutic benefits.
This summit is jam-packed with three days of actionable case studies, interactive workshops, and thought-provoking panel discussions, empowering you to tackle immunogenicity challenges in the ‘here and now’ and position your programs for long-term success.
Event in Numbers
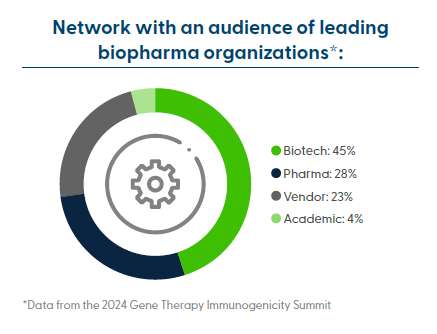
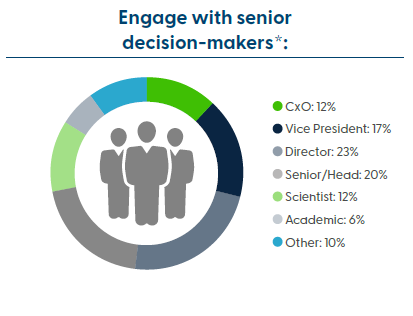
Audience Summary
This summit brings together professionals from preclinical, clinical, translational, bioanalytical, toxicology, and pharmacology development to get the latest insights into overcoming immunogenicity bottlenecks in gene therapy development.
R&D – Discover and digest the latest and greatest in ensuring the safety and efficacy of gene therapies to gain actionable insights for your gene therapy development efforts.
Business Development – Engage with other business leaders from 40+ industry groups to set up collaborations, secure investment, and unlock future company deals.
Service Provider – We provide unique networking opportunities for leading vendors in the gene therapy field to win big business and out pace their competitors. Check out our partnership opportunities here
Academia – You will be able to leverage the latest data and tech developments from the industry into your own research and network with industry leaders in the field.